


Chapter 21 Lecture 20 - Angular Momentum in Atoms and Molecules
we recall that
L2Ylm(q ,f )=l(l+1)2Ylm(q ,f )
21.1 Diatomic Molecules
such as H2 , O2 , CO , HCl , etc
we can treat this as a rigid rotor where R is a constant
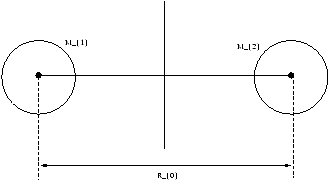
Figure 20.1 - Rotor Model
the moment of inertia about the centre of mass is
classically the energy of rotation is
we can quantise this in the following way
Thus the rotational energy eigenstates are the eigenstates of L2
| HrotYlm(q ,f )= |
|
Ylm(q ,f ) |
I=1.26× 10-46kgm2
l=1Þ E1=5.7× 10-25J
l=2Þ E2=1.7× 10-22J
the transistions between rotational states may occur, for example when l=1® 2 in an O2 molecule
D E=1.13× 10-22JÞn =1.7× 1011Hz
this is a frequency that lies in the far infrared or microwave region.
21.2 Central Potentials - Atoms
A central potential is spherically symmetric (definite parity)
V(r,q ,f )® V(r)
it reduces to a function in terms of only r , thus [P,H]=0 and also [P,L2]=[P,Lz]=0 .
The eigenstates of parity are also eigenstates of H , L2 and Lz . We wish to verifty that in this case H , L2 and Lz are compatible
we must use spherical polar for Ñ2
|
Ñ2º |
|
+ |
|
|
+ |
|
æ
ç
ç
è |
|
+cotq |
|
+ |
|
|
ö
÷
÷
ø |
(21.6) |
| |
|
+ |
|
|
ºPr2=[radial momentum]2 |
| |
|
+cotq |
|
+ |
|
|
º - |
|
=[angular momentum]2 |
|
H= |
|
æ
ç
ç
è |
|
+ |
|
|
ö
÷
÷
ø |
+ |
|
+V(r) (21.7) |
| |
|
æ
ç
ç
è |
|
+ |
|
|
ö
÷
÷
ø |
=radial kinetic energy (µpr2) |
| |
|
=angular kinetic energy (µL2) |
V(r)=potential energy
|
[H,L2]= |
|
[pr2,L2]+ |
|
[L2,L2]+[V(r),L2] (21.8) |
V(r) in a central potential depends only on r
[V(r),L2]=0
likewise because pr2 depends only on r
[pr2,L2]=0
[H,L2]=0 (21.9)
The energy and total angular momentum is compatible (also with Lz ).
Energy eigenstates are simultaneous eigenstates of L2 and Lz for a central point.
By using equation IV.50 we have
|
|
é
ê
ê
ë |
- |
|
æ
ç
ç
è |
|
+ |
|
|
ö
÷
÷
ø |
+ |
|
+V(r) |
ù
ú
ú
û |
Y (r,q ,f )=EY (r,q ,f ) (21.10) |
Y (r,q ,f )=R(r)Ylm(q ,f ) (21.11)
we know that
L2R(r)Ylm(q ,f )=l(l+1)2R(r)Ylm(q ,f ) (21.12)
and so equation IV.53 can be simplified
|
|
é
ê
ê
ë |
- |
|
æ
ç
ç
è |
|
+ |
|
|
- |
|
ö
÷
÷
ø |
+V(r) |
ù
ú
ú
û |
R(r)=ER(r) (21.13) |
-
this radial Schrödinger equation determines the energy E and R(r) for any system with a central potential
- Spherical Harmonics are universal for all such systems, for example nuclear and atomic wavefunction systems
- for V(r)=-r2/4pe0z/r the coulomb potential for hydrogen










