


Chapter 4 Two Electron Systems
Figure 5.1 - ???
r1Þ r1,q1 ,f1
r2Þ r2,q2 ,f2
r12=|r1+r2|
-
He :
- the nucleus is made up of either:
- Li+ :
- the nucleus is made up of 3p++3n
however Muonic Lithium is a neutral atom where 1e- is replaced with a muon ( µ- ). A muon has mass mµ=207me and the orbit is much closer to the nucleus
the muon shields two electrons from the nucleus, such as the two electrons in a Z=2 core.
4.1 The Schrödinger Equation for a Two Electron System
|
|
æ
ç
ç
è |
- |
|
|
- |
|
|
- |
|
|
- |
|
|
+ |
|
|
ö
÷
÷
ø |
Y =EY (4.1) |
| |
æ
ç
ç
è |
- |
|
- |
|
- |
|
- |
|
+ |
|
ö
÷
÷
ø |
Y =EY |
(H1+H2+H')Y =EY (4.2)
where
and similarly for the second electron. Also
4.2 Wavefunctions
If H' was small we could neglect it and attempt to write a zero-order wavefunction as
where Ynlml is the hydrogenic wavefunction with Z=2 . Then by seperating equation 5.1 into two independent hydrogen Schrödinger Equations for the electrons. The Recipe is to substitute equation 5.5 into equation 5.2 with H'=0 . We now premultiply by Y* and integrate by dV . We use
H1Yn=EnYn
H2Yn'=En'Yn'
Þ E0=En+En' (4.6)
the zero order leads us to the independent particle approximation.
4.3 Ground State of Helium
the zero-order wavefunction
Ys=Y1s(1)Y1s(2) (4.7)
|
Y1s=2 |
æ
ç
ç
è |
|
ö
÷
÷
ø |
|
e-2r (4.8) |
E0=E1+E1=2E1
however Enµ Z2 and Z=2 therefore
E0=2× (2)2E1 (for hydrogen)
E0=8E1=-108.8eV
this is the binding energy (the energy required to strip off both electrons leaving He++ )
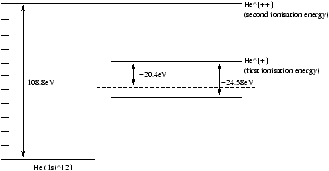
Figure 5.2 - Ionisation Energies of Helium
From figure 5.2 we find that the independent particle model is effectively useless!
-
Spectroscopic Notation:
- Two electrons in a combined state each in a 1s hydrogenic orbital, we write this as 1s2 or (1s)2 .
4.3.1 Beyond the Independent Particle Approximation - General Solution
We try Perturbation Theory
with
however <1/r12> is òò dr1dr2 . We must express 1/r12 in terms of r1 and r2 . This gives a very messy bit of calculus. However the answer is
| D E |
|
= |
|
Z atomic units~ 34eV |
| E=E0+D E |
|
=-108.8+34=74.8eV |
Egeneral solution He(1)=-20.4eV
where the (1) is the first order perturbation theory. This is not too bad considering that H' is not small. Variational calculations would be better.
4.4 Excited States of Helium
We assume that only one electron gets excited. We will try to obtain a wavefunction that is of the form
|
Y12=Y1s(1)Y |
|
(2) (n 1) (4.10) |
|
E(0)=- |
|
æ
ç
ç
è |
1+ |
|
ö
÷
÷
ø |
a.u. (4.11) |
Figure 5.3 - ???
The explanation gives two states at -4.8eV and -4.0eV (with respect to the He+ independent particle).
4.4.1 Beyond the Independent Particle Approximation
We will try Perturbation Theory as we will expect better results than for the ground state since the percentage in the discrepancy is smaller. The inner electon shields the outer electrons from seeing the full nuclear charge, so from Perturbation Theory
| D E= |
ó
õó
õ |
Y1s*(1)Y |
|
(2) |
|
Y1s(1)Ynlm(2)dr1dr2 |
| D E= |
ó
õó
õ |
|Y1s(1)|2|Y |
|
(2)|2 |
|
dr1r2 |
4.4.2 Identical Particles
Electrons are identical, which has not been accounted for properly in our throries. So for identical particles e1 and e2 should be indisguishable frin Y21 in every way.
If we consider hydrogen
where P1 is the probability that the electron is at r , q and f which can be interpreted as a charge cloud. Now we consider Helium
where P2 is the probability that the first electron is at r1, q1,f1 and the seond electon is at r2 , q2 and f2 which can be interpreted as a joint probability distribution. In principle we could measure this. However are |Y12|2 and |Y21|2 the same?
When normalised and symmetrised properly, the approximate wavefunctions for the excited states of Helium may be written as
| YS= |
|
(Y1s(1)Y |
|
(2)+Y1s(2)Y |
|
(1)) |
| YA= |
|
(Y1s(1)Y |
|
(2)-Y1s(2)Y |
|
(1)) |
(H1+H2)YA,S=E(0)YA,S
where
4.5 Perturbation Theory Using Psi-A and Psi-S
| D ES= |
|
ó
õó
õ (Y1s*(1)Ynlm*(2)+Y1s*(2)Ynlm*(1)) |
|
(Y1s(1)Ynlm(2)+Y1s(2)Ynlm(1))dr1dr2 |
| D ES= |
ó
õó
õ |Y1s(1)|2|Ynlm(2)|2 |
|
dr1dr |
2+ |
ó
õó
õY1s*(1)Ynlm*(2)Y1s(2)Ynlm(1) |
|
dr1dr2 |
D ES=J+K
also we similarly find
D EA=J-K
where J is the J integral we obtained from Perturbation Theory whilst using Y12 and Y21 . K is known as the exchange integral.
The explicit calculation of J and K gives
-
J and K are both positive
- J>K
- J and K depends on n and l (however not on ml ) so we can write them as Jnl and Knl
we note that
-
J is the Coulomb interaction betwen 1s and the nl charge clouds. This describes the shielding effect
- K is a measure of the likelyhood that an exchange might occur. It describes the overlap between the 1s and nl where the states are weighted by 1/r12
- ES>EA :
-
YS :
- tells us that the electrons huddle and so we see an extra electron repulsion which leads to a higher energy
- YA :
- the electrons avoid one another and so the energy drops as there is less electron repulsion
- the configurations have different energies, E=Enl . In Helium we see the degeneracy with respect to l is lifted for a potential which is not simply proportional to 1/r
- under high resolution YA splits into three states. This is a relativistic effect that depends upon spin
4.6 The Inclusion of Spin
s=1/2 for an electron. However we can have
Figure 5.4 -
l.
s Forces the Use of
jm ,
l and
s Pictures
by an analogy with j=l+s , we write
S=s1+s2
where S is the total spin of a two electron system.
where cSMS is a two electron spinor
S=s1+s2, s1+s2-1, ... ,|s1-s2|=1, 0
mS=s,s-1,... ,-s
so for S=1 we get mS=1,0,-1 . For S=0 we obtain mS=0 .
4.6.1 Two Electon Spinors
we try simple product states
c1=a (1)a (2)Þ
we guess that S=1 and mS=1 for c1 . This turns out to be a good guess
c2=a (1)b (2)Þ ¯
c3=b (1)a (2)Þ ¯
c4=b (1)b (2)Þ ¯¯
we guess that S=1 and mS=-1 for c4 , again we find this is correct.
We note that we have guessed S and mS . We can prove this by using operator methods (B+J, section 6.2).
c1 and c4 are symmetric under an exchange. c2 and c3 can neither be symmetric nor anti-symmetric.
The exchange of 1 and 2 in c2 to c3 , it can be shown (B+J) that c2 and c3 are not eigenfunctions of S2 .
Formal Quantum Mechanics (with matrix mechanics) tell us that c2 and c3 are unacceptable. c must be either symmetric or anti-symetric. However c2 and c3 can be used to construct a pair of acceptable spinors.
we now group all the acceptable c 's by S so that we can get the overall spin. So for S=0 we have a singlet and so c is anti-symmetric ( S=0 and MS=0 ). However for S=1 we get a triplet and so c+ , c1 and c4 are symmetric ( S=1 and MS=0,1,-1 respectivly).
4.7 The Pauli Exclusion Principle
We write that the total wavefunction as ( Ytotal )
Ytotal=YA,Sc
-
Dirac/Heisenberg:
- for a system of identical particles Ytotal must be either symmetric or anti-symmetric under an exchange
So a given species of particle has either Ytotal to be either symmetric or anti-symmetric.
Relativistic Quantum Field Theory gives us that for particles with spin:
-
1/2 :
- we get Fermions, for example an electron
- integral:
- we get Bosons
For a system of electrons Ytotal must be anti-symmetric under an exchange.
This is the Pauli Exclusion Principle (in disguise, see later for the normal statement of the Pauli Exclusion Principle).
Ytotal can be anti-symmetric in two ways
-
YAc+ , YAc1 and YAc4 where S=1 (so it forms a triplet)
- YSc- where S=0 (so it forms a singlet)
4.8 Spectrum of Helium
Spectroscopic notation wil be used, upper case letters signify the angular momentum of the whole atom.
(nl,n'l')2S+1L
for example the first excited state of Helium is
(1s 2s)1S or (1s 2s)3S
(1s 2p)1P or (1s 2p)3P
we note that for Helium Lº l since only one electron is excited. Also we notice that the ground state of Helium is
(1s)2'S
this means that there is no 3S state for the ground state since there is no YA for the ground state. The configuration splits into terms (eg. (1s 2s) , 1S and 3S ).
By the exchange effect the energy of the term depends on the spin. This is because of the Pauli Exclusion Principle and has nothing to do with relativistic effects.
-
Selection Rule:
- D S=0 , ie. the electric dipole operator does not act on the spin so the spin does not change
The consequences of this are
-
no singlet to triplet transistions are permitted, this is why we have parrallel and orthogonal helium
- (1s 2s)3S is metastable













