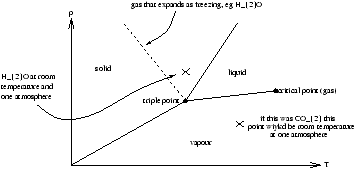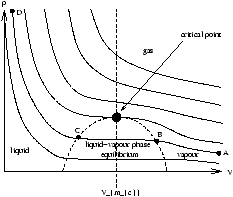
Figure 3.1 - A Real Gas (with lines of isotherms)
In the liquid-vapour equilibrium region volume reduces (increases) sharply at constant temperature and pressure as the vapour condenses into a liquid (or the liquid evaporates).
Although the temperature is constant there is a release (absorption) of heat